From September 1st to 4th, 2025, the US Food and Drug Administration (FDA) successfully completed a four-day on-site factory inspection and audit of Shanghai Hubang Intelligent Rehabilitation Equipment Co., Ltd.'s headquarters and production base in Shanghai. This high-standard factory inspection is a globally recognized gold standard in the medical device industry, directly verifying whether manufacturers continue to meet product safety and effectiveness requirements, and determining whether the company can obtain a key "passport" to the US market.

▲Group photo of Hubang with the US Food and Drug Administration (FDA)▲
Currently, the FDA is enforcing increasingly stringent quality supervision standards globally, particularly setting extremely high requirements for the integrity, traceability, and continuous compliance capabilities of product quality systems. Enterprises must establish a robust quality system that can withstand systematic and in-depth comprehensive inspections. FDA reviewers strictly adhere to quality system regulations and conduct systematic and in-depth assessments of various aspects such as organizational structure and management, personnel qualifications and training, CAPA system, comprehensive quality management, supplier and raw material control, full-chain control of the production process, and product packaging, storage, and transportation through various methods such as document review, on-site observation, personnel interviews, and record tracing.
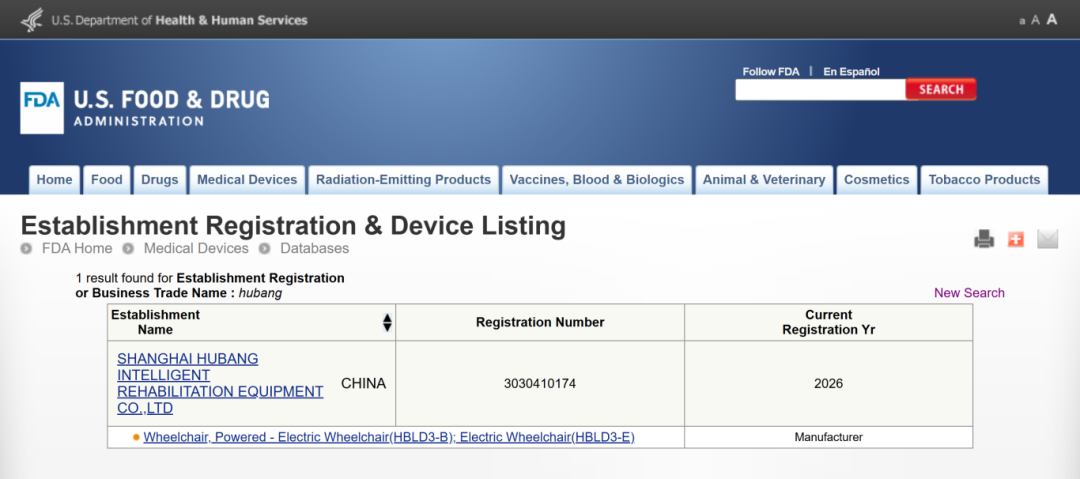
▲FDA registration and filing information of Shanghai Hubang Intelligent Rehabilitation Equipment Co., Ltd.▲
The efficient organizational skills, candid communication attitude, and solid daily management capabilities demonstrated by the Hubang team made the entire factory inspection process smooth and efficient. At the factory inspection summary meeting, the FDA inspectors highly praised and provided positive feedback on Hubang's mature and robust quality system, excellent on-site management capabilities, and the profound compliance literacy of all employees. They clearly stated that they looked forward to witnessing Hubang's excellent products serving more users in the global market in the future.
The successful completion of this factory inspection serves as the most convincing global endorsement for Hubang's long-standing commitment to adhering to the highest international standards, deepening its professional capabilities in the rehabilitation field, and dedicating itself to providing safe and effective products, all of which embody its core value proposition.



Hubang's successful passing of the rigorous FDA factory inspection this time is a strong testament to the rise of China's high-end medical device manufacturing capabilities. It demonstrates that leading Chinese enterprises have been able to systematically establish and operate a complete quality system that meets the world's top regulatory requirements, achieving a leap from "meeting standards" to "pursuing excellence". This not only opens up markets for individual products, but also signifies that the overall manufacturing level, management philosophy, and international integration of China's medical device industry have reached new heights, playing an increasingly important role in the global value chain. Hubang will take this successful FDA factory inspection as a new starting point, continue to hold itself to the highest international standards, and provide safer, more reliable, and more intelligent rehabilitation solutions for global users.








